“Respiratory syncytial (sin-SISH-uhl) virus, or RSV, is a common respiratory virus that usually causes mild, cold-like symptoms. Most people recover in a week or two, but RSV can be serious. Infants and older adults are more likely to develop severe RSV and need hospitalization.” [1]
RSV can also cause more severe infections such as bronchiolitis and pneumonia. It is the most common cause of bronchiolitis and pneumonia in children younger than 1 year of age.
Each year in the United States, RSV leads to approximately:
2.1 million outpatient (non-hospitalization) visits among children younger than 5 years old.
58,000-80,000 hospitalizations among children younger than 5 years old.
60,000-160,000 hospitalizations among adults 65 years and older.
6,000-10,000 deaths among adults 65 years and older.
100–300 deaths in children younger than 5 years old. [2]
I will interject here that no one is downplaying the severity or prevalence of RSV. We all know someone with a baby who needed hospitalization and how dangerous it can be especially for young children. I’ve only recently started hearing about its prevalence among the elderly but obviously, it can be horrible for them as well.
RSV has an interesting origin story. It begins with a paper published in 1956, detailing the discovery of a virus causing coughing, sneezing, and mucopurulent discharge in a group of 20 chimpanzees at the Walter Reed Army Research laboratory and goes on to discuss a laboratory worker who experienced the same symptoms by which they discovered an etiological association between the chimpanzee coryza virus (CCA) and the infection of several human beings. [3]
The next year we see a study published reporting on two isolations of similar agents from infants with severe lower respiratory illness (bronchopneumonia, bronchiolitis and laryngotracheobronchitis). [4] The two viruses were indistinguishable from an agent associated with the outbreak of coryza in chimpanzees (CCA virus) studied by Morris et al.
This paper is where they first note “syncytial areas” (photos included, see reference 4) in the cells. From what I can gather, there was cellular clumping or fusion and this is called “syncytia”. They talk about how distinct this virus was in this regard, “Of the viruses which infect human beings only measles, mumps and CA viruses have been previously shown to produce syncytial changes in cultures of various types. Measles virus produces syncytial changes in KB cultures but at least 5 weeks are required for complete cell destruction whereas Long and Snyder viruses destroy the cell sheet within 3 to 6 days.” [5]
Around this time CCA (chimpanzee coryza agent) was named RSV (respiratory syncytial virus). [6]
Because of the SV-40 debacle, many people already know that monkeys are used in the production of both oral [7] and inactivated polio vaccines. [8] I am only now learning about the CCA/RSV contamination association, however, and I’ve been researching the topic of vaccine problems for over 6 years now. It is a topic quite difficult to amass any data on but I’ve made a valiant effort to do just that, here.
“Prior to July 1960, the influenza and parainfluenza viruses predominated in infant epidemic respiratory infections; in July 1961 the pattern changed abruptly with sudden increase in bronchiolitis and bronchitis, infrequent before. 58% were under 12 months, and patients under 4 years predominated.” [9]
“RSV has spread via contaminated polio vaccines like a wildfire all over the world and continues causing serious lower respiratory tract infections in infants.” [10]
Moving on to vaccines for this virus we come across a long and sordid history of failed attempts. As early as 1968, we have the infamous study where we compare an aluminum based, inactivated RSV vaccine to a “control group” vaccinated with a parainfluenza vaccine. The children receiving the RSV vaccine saw a four-fold increase in all the right antibodies and all was well until the virus began circulating in the community, and then they saw “enhanced disease” in the children who contracted RSV. 80% were hospitalized compared to only 5% in the control group. The vaccine actually primed them to have a worse time with the disease than if they had been left unvaccinated. There were 2 deaths.
“It seems clear that infants who received this vaccine were not protected against natural infection and also, when they became naturally infected their illness was more severe than that seen in cohorts…” [11]
Three other studies done around that time revealed the same results. [12, 13, 14]
The epic failure of inactivated vaccines led scientists to abandon them in favor of live attenuated vaccines but they “…have thus far proven to be poorly immunogenic or underattenuated, resulting in upper airway obstruction from increased production of mucus and secretions.” [15]
The reason for may be because although these vaccines effectively produce serum antibody levels seemingly adequate for protection, they do not produce antibodies in the respiratory tract and since that is the mode of transmission and the virus is first encountered through nasal passages and the lungs, the serum antibodies are useless.
On top of that problem, young infants do not respond even serologically the same way as older children/adults. “Of the children given a live candidate RSV vaccine, less than 40% of those younger than 5 months produced a detectable, but transient, cytotoxic T-cell response to RSV, compared with 65% of those 6 to 24 months of age. RSV immunization in early life is further complicated by the apparent bias toward a Th2-type immunologic response to RSV that exists during the first 3 months of life.” [16]
Before we go over the vaccines targeting RSV on the market right now I just need to preface this section with the acknowledgement that these are very new in terms of having any types of postmarking surveillance data which is very important as these are put on the market while clinical trails are still in effect after only 6 months of monitoring for safety which is the best they can do these days.
We currently have a vaccine for those over 60 years of age by GSK called AREXVY. Of the 3 clinical trials done before licensure, only one had a saline placebo and the longest any group was followed for safety was 6 months.
This is an inactivated vaccine.
The virus used in the vaccine is grown on Chinese Hamster Ovary cell lines and “each 0.5-mL dose is formulated to contain 120 mcg of the recombinant RSVPreF3 antigen, 25 mcg of MPL, and 25 mcg of QS-21. Each dose also contains 14.7 mg of Trehalose, 4.4 mg of sodium chloride, 0.83 mg of potassium dihydrogen phosphate, 0.26 mg of dipotassium phosphate, 0.18 mg of polysorbate 80, 0.15 mg of disodium phosphate anhydrous, 0.5 mg of DOPC, and 0.125 mg of cholesterol. AREXVY contains no preservative. Each dose may also contain residual amounts of host cell proteins (≤2.0%) and DNA (≤0.80 ng/mg) from the manufacturing process.” [17]
According to the current insert (see reference 17), the phase 3 clinical trials are still monitoring for efficacy which at this time is a median of 6.7 months although they are planned to be followed for up to 36 months. In that time, the reported efficacy for this vaccine is 82.6%.
I am seeing no evidence in the insert or elsewhere proving that the problem of disease enhancement has been completely ruled out.
This vaccine uses an adjuvant that I haven’t researched very much but a short description is, “AS01 is a liposome-based adjuvant that contains two immunostimulants (MPL and QS-21). A series of experiments in mice determined that AS01 induces a rapid and transient innate immune response at injected site and the draining lymph node.” [18]
The latest vaccine on the market targeting RSV is ABRYSVO by Pfizer, given to pregnant women at 32-36 weeks gestation. This ad kind of says it all, but let’s get into the details anyway.
This is a recombinant inactivated subunit protein vaccine (more on that later).
Under the “warnings and precautions” section of the insert we come across the heading “potential risk of preterm birth.” It goes on to say, “A numerical imbalance in preterm births in ABRYSVO recipients was observed compared to placebo recipients in two clinical studies. To avoid the potential risk of preterm birth with use of ABRYSVO before 32 weeks of gestation, administer ABRYSVO as indicated in pregnant individuals at 32 through 36 weeks gestational age. Pregnant individuals who were at increased risk of preterm birth were generally excluded from clinical studies of ABRYSVO.” [19]
So to avoid having your baby before 32 weeks, avoid getting the vaccine before 32 weeks. Got it. Also, individuals at increased risk of preterm birth were excluded from the clinical trials so there’s that. Of course, they still say the data is insufficient to establish or exclude a causal relationship but that’s according to plan, if you ask me. If your clinical trial is in any way ambiguous, you can still get it approved and once it’s approved you’re off the hook and don’t have to worry about pesky stuff like properly powered studies with an actual placebo control because then it becomes unethical to withhold this amazing product from any group of children and the circle of “settled science” becomes complete.
When studying pregnant women in these trials, instead of using a saline placebo for the control group, they give the entire vaccine except for the RSV component (antigen). In what world this is considered ethical behavior I couldn’t tell you.
“In Study 1, adverse events in infants from birth to 1 month of age were observed in 37.1% in the ABRYSVO group compared to 34.5% in the placebo group. Low birth weight was observed in 5.1% of participants in the ABRYSVO group versus 4.4% in the placebo group, and neonatal jaundice was observed in 7.2% in the ABRYSVO group versus 6.7% in the placebo group.”
“There were 10 (0.3%) fetal deaths in the ABRYSVO group and 8 (0.2%) in the placebo group. Among the infants born to individuals in the ABRYSVO group and in the placebo group, 202 (5.7%) and 169 (4.7%), respectively, were born preterm.”
I just have to say that since the placebo group got all of the ingredients in the vaccine besides the RSV component and the safety of those ingredients injected into pregnant women have not been studied, it’s very difficult to make any real conclusions from this data other than seeing that it was deliberately skewed from the beginning.
The plan is to monitor the babies born in year 1 for 24 months and those born in year 2 will be monitored for 12 months. At time of data collection the median time spent monitoring is 8.9 months.
The virus used in this vaccine is also grown on Chinese Hamster ovary cell lines and “is formulated to contain 120 mcg of RSV stabilized prefusion F proteins (RSV) per 0.5 mL. ABRYSVO also contains the following buffer ingredients: 0.11 mg tromethamine, 1.04 mg tromethamine hydrochloride, 11.3 mg sucrose, 22.5 mg mannitol, 0.08 mg polysorbate 80, and 1.1 mg sodium chloride per 0.5 mL. ABRYSVO contains no preservatives. Each dose may also contain residual amounts of host cell proteins (≤0.1% w/w) and DNA (<0.4 ng/mg of total protein) from the manufacturing process.”
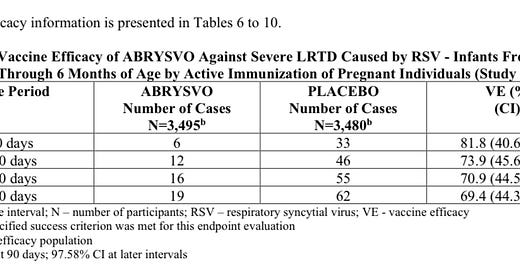
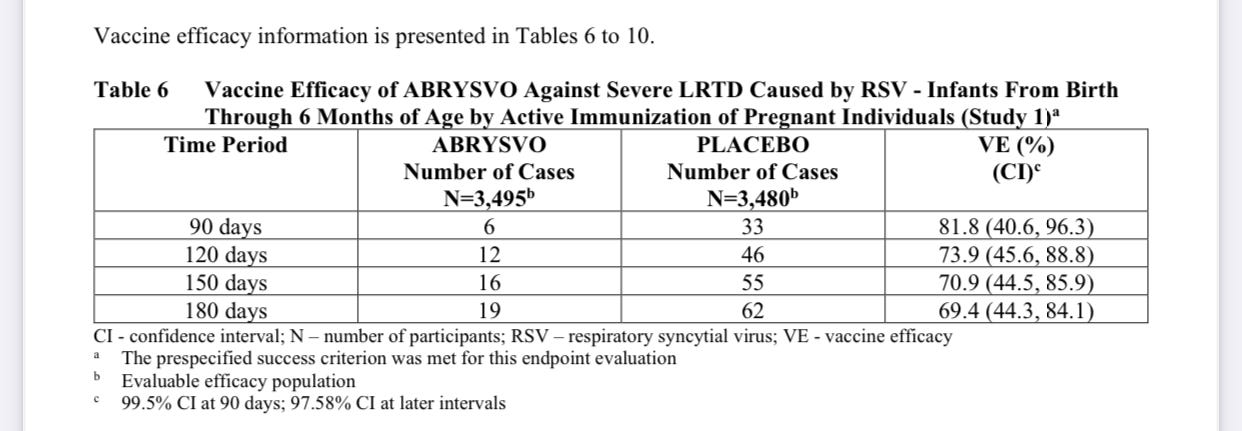
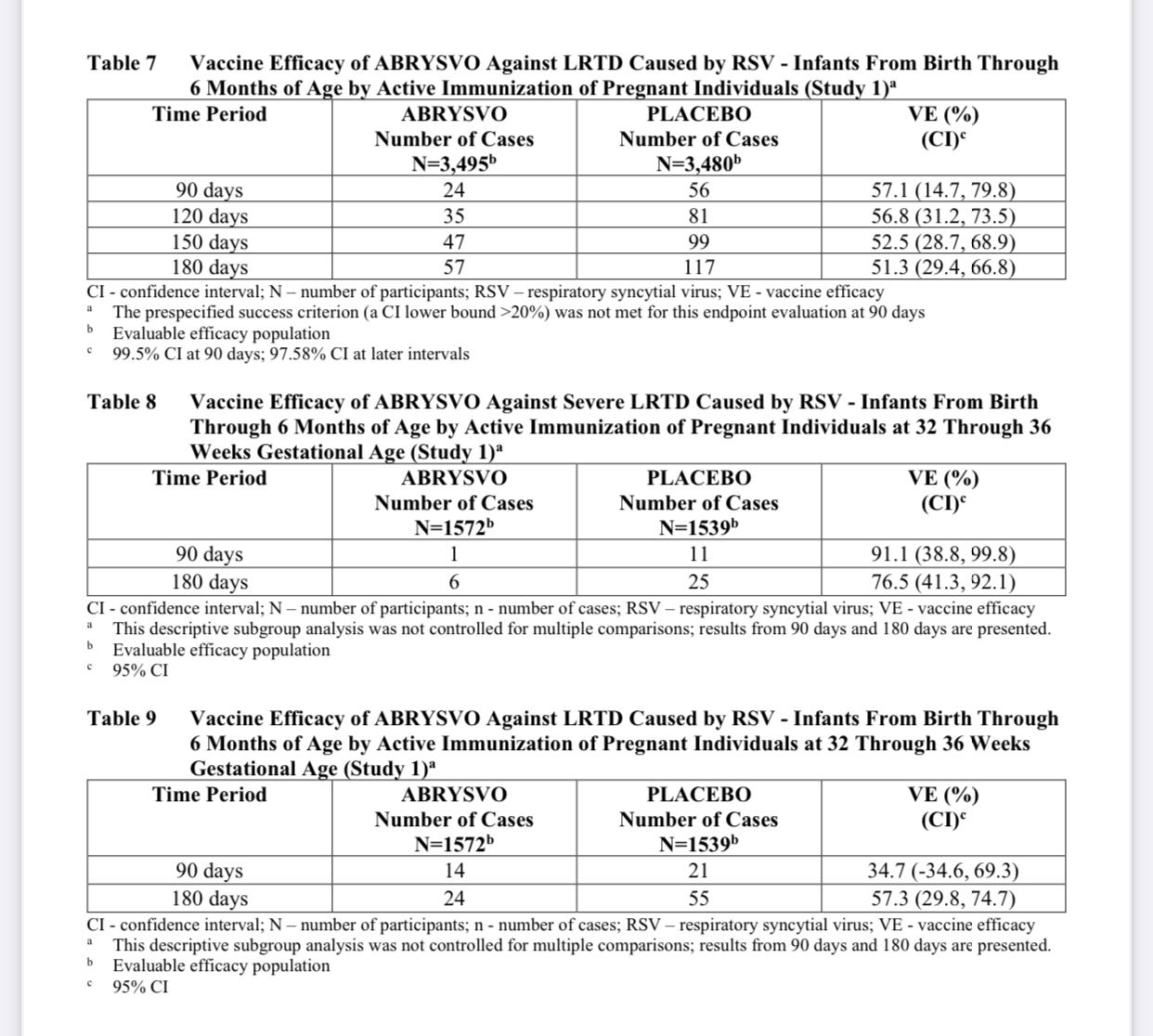
Getting into vaccine efficacy: “The VE (vaccine efficacy) results met the statistical criterion for success (a CI lower bound >20%) for reducing severe LRTD (lower respiratory tract disease) due to RSV, at all timepoints to within 180 days. The VE results did not meet the statistical criterion for success (a CI lower bound >20%) for reducing LRTD due to RSV; however, clinically meaningful efficacy was observed after 90 days through 180 days after birth.”
It appears to be more effective in those first 90 days against severe disease. But even then, it is a bit lackluster.
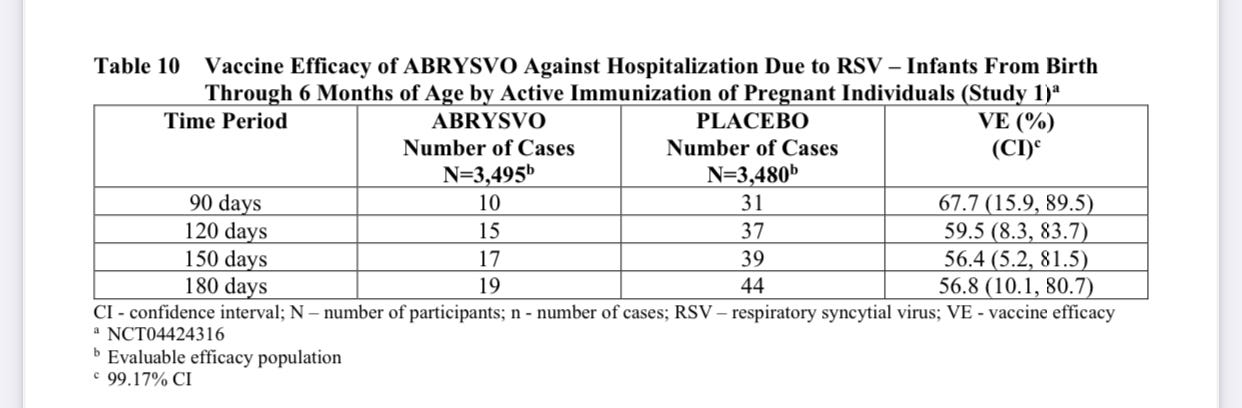
I’m not sure how a vaccine can be 81% effective at preventing severe disease but only 67% effective at preventing hospitalization.
This type of vaccine isn’t exactly new but it is the first time we’re using this type of vaccine in pregnant women. Whole-cell vaccines contain an inactivated version of the entire pathogen (virus or bacteria). Subunit vaccines include only parts of the virus or bacteria that contain the antigens needed to stimulate an immune response, but do not include all the other molecules in the virus or bacteria.
“Since therapeutic proteins have a well-established history of formulation development and use in humans, it was assumed that their transformation to vaccines would be relatively straightforward. This has turned out not be the case, however.” [20]
Inefficacy seems to be a chronic problem with these vaccines, “However, some problems remain to be solved, such as low immunogenicity and humoral immunity bias. Adjuvants can effectively enhance and adjust vaccine immune responses.” [21]
If an adjuvant is necessary for enhanced effectiveness, I’m not seeing one in the ingredient list above. Not that I’m complaining. I do have to note the absence of aluminum in both of these new vaccines which I find encouraging although I’m still skeptical of the other adjuvant in AREXVY.
Incidentally, GSK had been running THREE phase 3 clinical trails on pregnant women with their vaccine for RSV and actually halted all 3 because of a 37% increased risk of preterm birth in one of the trials. This is the exact same type of vaccine Pfizer and the CDC are recommending to pregnant mothers. It had an efficacy rate of 65.5%-69%. [22]
“In a document submitted to the FDA, GSK’s data showed 238 preterm births out of 3496 (6.8%) in the vaccine arm and 86 out of 1739 (4.9%) in the placebo arm—around one extra preterm birth for every 54 vaccinated mothers. There were 13 neonatal deaths in the vaccine arm and three in the placebo arm.” [23]
“According to GSK’s analysis, the difference in preterm births was highest in LMIC in women who had decided to have different additional vaccines, with 8.2% in the vaccine arm compared with 4.3% in the placebo arm. None other of the factors analysed could explain the safety signal, including SARS-CoV-2 infections.”
“Pfizer’s vaccine is similar to GSK’s. Both are subunit vaccines using a recombinant RSV F protein of the virus, stabilised in its prefusion state. “I can’t really give you an idea as to why one would cause a problem and the other one wouldn’t,” Meissner said.” [24]
I’m sure it has nothing to do with the fact that Pfizer is better at obfuscating the data or the fact that they have such a cozy relationship with the FDA or anything. Same vaccine but much less dangerous, even though no one can explain why. Nothing to cause concern, I’m sure.
On that note, the advisory committee from the FDA approving this vaccine voted 10-4 when asked if the data from the trials were sufficient to demonstrate the safety of the vaccine. If you don’t know the history of the unanimous vote when it comes to vaccines, you’ll miss the significance of 4 people having strong enough qualms with the safety data to vote no to that question but let me assure you, it’s a big deal to have even one person vote no on the safety of a vaccine when it comes to these committees.
But not to worry, Pfizer’s response to this is that the “safety concerns can be studied post-approval.” [25]
If you are in any way uncomfortable with the risks and possible lack of efficacy with these vaccines, let’s go over some possible alternatives (for children):
My children have never been diagnosed with RSV but since it is so prevalent, I assume they have all had it at some time or another. Isaac has been more prone to croup and one of those times may very well have been RSV. What helps the most in my experience is running a humidifier, vitamin c (thins and decreases mucous), cold air, and garlic salve mixed with essential oils like eucalyptus, peppermint, and lavender, rubbed on chest and then topped with a warm flannel, especially overnight.
In addition to this, breastfeeding is the best prevention in my opinion. This metanalysis concludes, “Results indicate that non-breastfeeding practices pose a significant risk for severe RSV-associated ALRI and hospitalisation. Exclusive breastfeeding for >4–6 months significantly lowered hospitalisation, length of stay, supplemental oxygen demand and admission to intensive care units. With both exclusive and partial breastfeeding benefiting infants who develop RSV-associated ALRI, breastfeeding should be promoted globally as an adjunct primary prevention.” [26]
https://sci-hub.se/https://pubmed.ncbi.nlm.nih.gov/13478578/
ibid
https://media.panaceabiotec.com/documents/2019/7/24/bOPV(PTB)-Package-Insert_.pdf
ibid
ibid
https://www.tandfonline.com/doi/full/10.1080/14760584.2016.1213632
https://www.sciencedirect.com/science/article/abs/pii/B9780128143575000015
https://www.frontiersin.org/journals/immunology/articles/10.3389/
https://www.medpagetoday.com/infectiousdisease/vaccines/109161#
ibid